HONG KONG, Aug. 24, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today released its 2023 interim results. Revenue in the first half of 2023 reached approximately RMB3,783.8 million, up 22.3% on a yearly basis. Gross profit was approximately RMB3,201.6 million, up 24.8 % year on year. The normalized earnings before interest, taxes, depreciation and amortization (EBITDA) were approximately RMB1,518million, an increase of 17.7% over the same period last year. Net profit attributable to owners of the parents rose by 20.1% year on year to RMB 1,191 million. In the future, 3SBio will fully leverage its advantages as an integrated platform for research and development (R&D), manufacturing, commercialization and external partnerships, and continuously foster new business growth drivers.
Core biopharmaceutical products posted robust performance with record sales
In 2022, the Company's biopharmaceutical products posted steady growth in revenue and maintained their leadership in the market. Sales of TPIAO, the world's only commercial recombinant human thrombopoietin (rhTPO) used to treat thrombocytopenia, totaled RMB2,019.1 million, a year-on-year increase of 28.2 %. Early this year, TPIAO achieved a renewal in the National Reimbursement Drug List (NRDL) and it was listed with the highest-level recommendation by the Chinese Society of Clinical Oncology (CSCO) Clinical Guidelines for the Diagnosis and Treatment of Cancer Therapy Induced Thrombocytopenia (CTIT) (2022 Edition).
As the leaders in the recombinant human erythropoietin (rhEPO) market, EPIAO and SEPO posted sales of RMB463.2 million in the first half of 2023, maintaining a dominant market share of 42.9%.
Sales of Yisaipu for the treatment of rheumatoid arthritis, ankylosing spondylitis and psoriasis were RMB 300million in the first half of 2023, up 25% year on year. The pre-filled injection of Yisaipu was approved for marketing in March this year.
Sales of Cipterbin for the treatment of HER2-positive metastatic breast cancer were RMB 108.6 million, an increase of 60.5% year on year. Additionally, the CSCO Clinical Guidelines for the Diagnosis and Treatment of Breast Cancer (2022 Edition) listed Cipterbin with the highest-level Class I recommendation for the treatment of patients with HER2-positive advanced breast cancer, and the number of hospitals and patients covered had a significant rise.
Hair health business posted fast growth with the help of digital marketing
Sales of Mandi (minoxidil tincture), an over-the-counter (OTC) product for the treatment of androgenetic alopecia and alopecia areata, maintained fast growth. In the first half of 2023, sales of Mandi were RMB495.5 million, representing a year-on-year increase of 35.3 %. As a scientific, effective, safe and convenient hair health solution, minoxidil has been increasingly recognized with its market size expanding year by year. As a leading brand of minoxidil, Mandi had a share of 70% in medical institutions, ranking first in the minoxidil market. In the first half of 2023, the Mandi team continued to bolster patient education and expand digital marketing. In addition to collaborating with leading digital platforms, the Company also leveraged new media operation platforms and explored e-commerce marketing channels. Sales through e-commerce channels jumped64% in the first half of 2023, giving full play to the market potential of the OTC product.
CDMO achieved accelerated growth
In the first half of 2023, the Company's CDMO business continued to expand, with revenue of RMB94.9 million, up 71.6 % year on year. The overseas and domestic sites posted revenue growth of 29% and 246%, respectively. Currently, the CDMO business has secured customer orders valued at more than RMB160 million, and differentiated CDMO positioning on commercial products has demonstrated impressive results.
Pipeline value constantly enhanced under accelerated clinical developments
In the first half of 2023, the Company had 30 product candidates within the active pipeline. Among them, there were 10 product candidates in hematology/oncology, 13 product candidates for auto-immune diseases and ophthalmology, 5 product candidates in nephrology and 2 product candidates in dermatology. Up to now, 10 candidates have been advanced to the clinical phase III or NDA-enabling phase, and 5 generic candidates are in the BE- and ANDA-enabling phase.
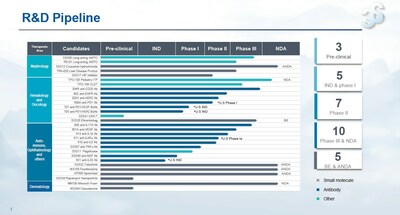
In the first half of 2023, two key products of 3SBio were approved for commercial launch. The prefilled injection of Yisaipu provides patients with an easy-to-use option and significantly improves the convenience of administration. Remitch (nalfuraphine hydrochloride orally disintegrating tablets) is the first drug marketed in China for the treatment of pruritus in dialysis patients, and the IND for the phase III clinical trial has been approved for treating pruritus in patients with chronic liver disease (only in cases where the efficacy of existing treatments is not desirable), which is expected to cover a larger patient population. The results from the phase II clinical trial of SSS06, the company in-house long-acting EPO, showed that a robust safety profile was ensured when the dosing interval was extended to 2 weeks. The phase III clinical trial has also seen the last patient out. The readout is expected in the second half of the year, and the NDA-enabling process will start.
Several candidates under Sunshine Guojian for the treatment of auto-immune diseases achieved development milestones in the first half of the year. The phase III clinical trial of anti-IL-17A mAb (608) for the treatment of plaque psoriasis has completed patient enrollment. The phase II clinical trials of anti-IL-1β mAb (613) for the treatment of acute gouty arthritis and IL-4R α mAb (611) for the treatment of atopic dermatitis, chronic sinusitis with nasal polyps and moderate to severe chronic obstructive pulmonary disease have both met the primary endpoints. The phase II clinical trial of anti-IL-5 mAb for the treatment of severe eosinophilic asthma has completed patient enrollment. The Company is expected to embrace a commercialization wave for its innovative products, and key pipeline products are expected to be advanced to commercialization every year in the future.
In addition, the IND-enabling process is underway for the bridging trial of Winlevi® as a acne treatment, on which the Company is collaborating with Cosmo Pharmaceuticals. As the first acne medication with a new mechanism of action approved by the FDA in 40 years, Winlevi® has been widely recognized by dermatologists in the U.S., and prescribed by 15,000 doctors since its launch in December 2021, with more than 670,000 prescriptions in total, making it the most prescribed acne medication in the U.S. It is expected to become the first marketed topical androgen receptor inhibitor to treat acne vulgaris in the Chinese mainland under the promotion of 3SBio, bringing a new treatment option to hundreds of millions of acne patients in China.
Looking ahead, 3SBio will continue to focus on the fields of its strength, namely nephrology, autoimmune diseases, hematology, oncology and hair and skin, while eyeing global opportunities for joint development of high-quality candidates. The Company will leverage both in-house research and development and external partnerships to make every effort to promote the early launch of new drugs with robust clinical value to address real-world patient needs.
Dr. Jing LOU, Chairman and CEO of 3SBio, commented: "In 2023, as we celebrate our 30th year, we remain committed to our mission of 'making innovative antibody drugs within reach.' Our diverse businesses are thriving and delivering steady performance growth. We will stay true to our aspiration and forge ahead to deliver on our mission. Looking ahead, we will continue to foster innovation in the areas of focus, actively advance all businesses, uphold our social responsibility, and bring forward more transformative biopharmaceuticals to market for the benefit of patients."
About 3SBio Inc.
3SBio is a leading bio-pharmaceutical company integrating research and development (R&D), manufacturing and commercialization, with a focus on improving the life quality of patients with high-quality medicines to benefit human health. At present, the Company owns more than 100 national invention patents and has launched more than 40 products into the market, covering several therapeutic areas such as nephrology, oncology, autoimmune diseases, ophthalmology and dermatology. The Company owns four R&D centers of the National Engineering Research Center of Antibody Medicine and dual platforms for biopharmaceutical and chemical medicine. The Company has a pipeline of 30 products under R&D, and 25 of them are the national new drugs. The Group also owns five production bases complying with the GMP standards. In the future, 3SBio will continue to uphold the concept of "Care for Life, Cherish Life, Create Life" to foster a world-leading biopharmaceutical company in China. Please visit www.3sbio.com for additional information.
Cautionary Note and Forward-Looking Statements
This press release contains forward-looking statements, such as those relating to business or products outlook, or Company's intent, plans, beliefs, expectation and strategies. These forward-looking statements are based on information currently available to the Company and are stated herein on the basis of the outlook at the time of this press release. They are based on certain expectations, assumptions and premises, some of which are subjective or beyond our control. These forward-looking statements may prove to be incorrect or may not be realized in the future. With respect to any new product or new indication, we cannot guarantee that we will be able to successfully develop or eventually launch and market such product or indication. Underlying the forward-looking statements is a large number of risks and uncertainties. Further information regarding such risks and uncertainties may be found in our other public disclosure documents. The scientific information involved may only be preliminary and empirical. Shareholders and potential investors of the Company are advised to exercise caution when dealing in the shares of the Company.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/3sbio-announces-2023-interim-results-with-revenue-growing-over-20-year-on-year-and-pipeline-value-constantly-enhanced-301909757.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/3sbio-announces-2023-interim-results-with-revenue-growing-over-20-year-on-year-and-pipeline-value-constantly-enhanced-301909757.html
SOURCE 3SBio Inc.


