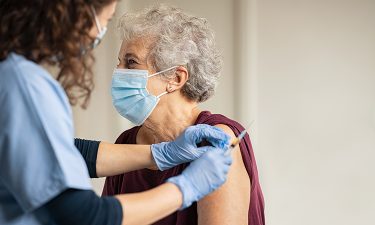
As most of the world sits in the grip of another devastating wave of the COVID-19 pandemic, vaccines are offering a glimmer of hope that some form of normality may well return soon.
In the
Although a commendable achievement, the speed at which these vaccines have been approved and passed by regulatory bodies has caused some concern. Consequently, scientists have moved quickly to allay fears that these vaccines have skipped any of the rigour that usually comes with vaccine approval.
However, there has been some confusion over whether the three vaccines approved in the
What problems can some food allergies cause with vaccines?
LR: The viruses used to make flu vaccinations are often grown in a hen's egg, although the amount of ovalbumin (egg protein) in the final vaccinations is minute and most people with an egg allergy can safely receive these vaccines.
Unlike most flu vaccinations, the COVID-19 vaccines are not grown in hen's egg. Therefore, the finished vaccine does not contain any traces of egg at all. Neither the
Are the three vaccines approved by the MHRA in the
LR: As the Medicines and Healthcare products Regulatory Agency (MHRA) has continued their close surveillance of the vaccine roll out, they have now advised that individuals with a history of allergy or anaphylaxis to any food can receive any COVID-19 vaccine, as long as they are not known to be allergic to any component (excipient) of the vaccine. None of the currently approved
What was the cause of the initial concern over the
LR: When the
As a consequence of the guidance revisions, our helpline has been incredibly busy because people are still very worried. We have been working hard to support individuals and healthcare professionals, through ensuring we have the most up to date information in our dedicated COVID-19 resource hub on our website.
Does the Anaphylaxis Campaign work with regulators and manufacturers when it comes to vaccines?
LR: We work closely with the MHRA , and if you go onto our website, you will find a wealth of information to help people with allergies and anaphylaxis navigate COVID-19 and having the vaccination.
We have worked closely with clinical experts at the
Would you advise somebody with a food allergy to get the vaccine?
LR: Having a food allergy is not a contraindication to receiving any of the currently approved
Biography

© Russell Publishing Limited, 2021. All Rights Reserved., source




